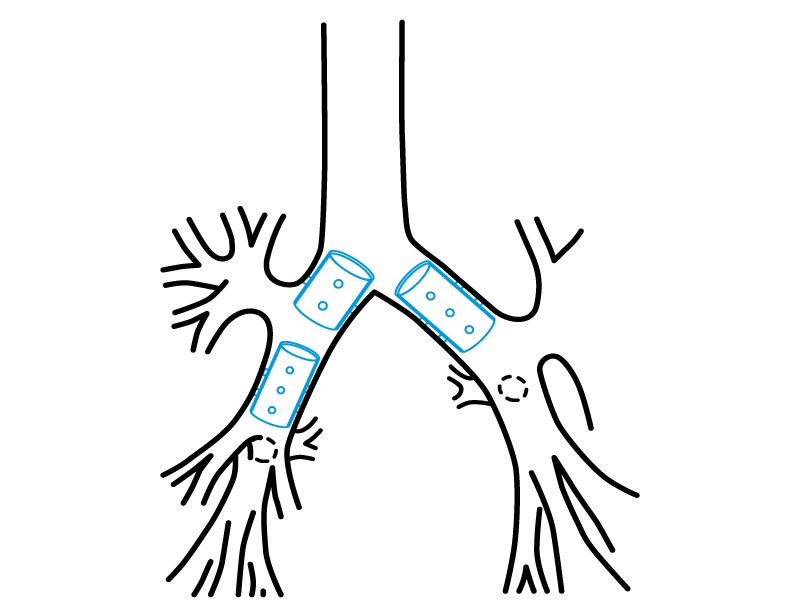
Stening® Bronchial Stent
Code ST

Description
Stening® Bronchial stent follows the tracheal stent general design, with some changes on its walls’ thickness and its dimensions. It is introduced in a wide range of diameters and lengths, sharing a lot of indications described for the tracheal version.
Indications:
- Bronchial neoplasms.
- Neoplasms that invade the tracheal carina or its slopes.
- Imminent atelectasis.
- After laser resection, cryotherapy or electrocautery, to maintain the airway opened.
- Bronchial stenosis.
- Post infectious stenosis (endobronchial tuberculosis, histoplasmosis mediastinal fibrosis, herpes virus, diphtheria).
- Post-traumatic stenosis.
- Post-surgical term-terminal bronchial anastomosis stenosis.
- Bronchial rupture.
- Extrinsic compression.
- Bronchomalacia.
- Amyloidosis.
- Excessive dynamic compression of the airway.
- Bronchus invasion caused by an oesophageal carcinoma.
- After endoscopic resection of bronchial metastasis.
- Stening HE with 1 mm thickness (thin wall with lower radial pressure).
- Stening SAP with 2 mm thickness (more robust wall, with high radial pressure)
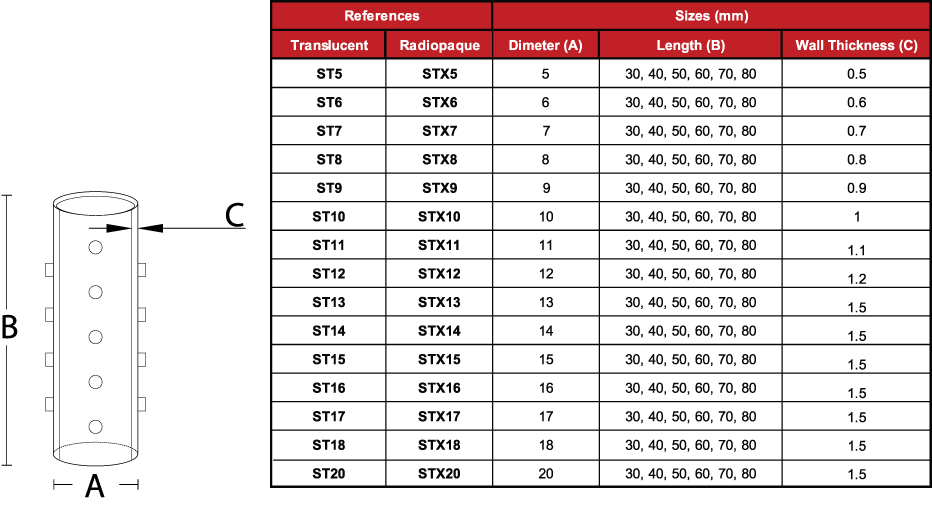
Due to the characteristics of the production process, the
measurements of the devices can vary by +/- 2%
- Medical grade silicone
- Bevelled edges to prevent granulomas
- Spur system to prevent migration
- Removable
- Surface of maximum softness to avoid adherence of secretions
- Transparent or Radiopaque
You can follow the technique of introduction and removal described for the Stening® Tracheal Stent, replacing the tracheoscope for a bronchoscope of a suitable diameter. In general terms, a bronchoscope with an external diameter of 11mm and 12mm can charge all the bronchial Stening® prostheses. In bronchoscopes with a diameter of 10 mm every Stening® Bronchial Stent can be introduced excepting for the ST13 and ST12 with a length bigger than 40mm.
The procedure will take place under general anaesthesia.
The implant of these type of prostheses can be made directly through the work channel of the tracheoscope or bronchoscope.
A conventional introducer for silicone prostheses can be used as well. A rigid endoscope will be used to access the airway.
The length and diameter of the area that the stent will cover must be established correctly.
If you want to know the length of the area, you can mark the tracheoscope when its extreme is located at the end of the injury. Repeat the process once the tracheoscope is moved up to the beginning of the injury. The diameter of the trachea or bronchi must be estimated by comparing it with the diameter of the endoscope used.
- Lubricate the introducer nozzle with lidocaine gel, preventing the lubricant from reaching the operator’s fingers.
- Fold the Stening® over its axial axis and put it inside the prostheses introducer through its nozzle.
- Remove the nozzle.
- Surpass the injured area with the tube of the tracheoscope and put its distal edge over the healthy mucosa, exceeding from 5 to 7 mm the affected zone.
- Put the introducer inside the tracheoscope.
- Press the ejector while the tracheoscope is removed and the ejector plunger progresses. That is, the plunger of the stents’ charger has to be pressed as the endoscope is removed.
In this way, the prosthesis will be released. If it is necessary, it can be moved to a better position with an alligator forceps. The manoeuvre is much easier when the stent is lower than the injury.
Steps 1 to 3 will be repeated.
You have to stop the tracheoscope that contains the introducer and the prosthesis 5mm before the injury.
After that, you have to press the ejector plunger slowly. By doing this, the prosthesis will be expelled into the injured trachea.
Some stents’ charger models are not introduced inside the tracheoscope, but they are added to the proximal extreme, from where the stent is expelled. To accomplish this, the endoscope will have to be stopped in a proximal or distal way to the injury, as it was explained before, to push the prosthesis with the plunger that is provided with the endoscopic instrumental. In this way, the stent will travel through the tracheoscope until it reaches the trachea. At this point, we will feel a small reduction of the resistance in the pressure performed on the plunger, showing that the stent has begun to abandon the endoscope interior.
The stent may require some additional manoeuvres, focusing on correcting or adjusting its final position.
It is preferable to correct stent that has been installed beyond the desired position than the opposite, due to the arduousness of pushing a stent that has been released before the damaged zone.
To move a stent in a proximal way, it can be taken by its edge and then you must pull with kindness.
To be more accurate we recommend a manoeuvre that consists on taking the stent by its edge as it was mentioned before. After that, you have to advance with the optical vision through the stent’s inside until you see its ending. Now pull the forceps and you will be able to see how the stent ascends through the airway.
Stop the pulling as long as you consider that the position is correct.
We will proceed to the intubation with the tracheoscope or the rigid bronchoscope depending on the case.
Of easy extraction, the silicone stent must be taken by its proximal edge with a crocodile forceps, with enough steadiness. Rotate the forceps 360º so that the stent bends, acquiring an omega shape and losing its radial resistance to compression. Then, pull the forceps and extract the prosthesis together with the tracheoscope.
The stent proximal edge can be introduced inside the tracheoscope. With this manoeuvre the vocal cords are protected during the extraction.
Other implant and removal methods are also possible depending on the experiences and preferences of the surgeon.
Maintain the moisture of secretions whenever they appear, by taking nebulisations frequently with a warm isotonic saline solution.
Perform a periodic check-up following your doctor’s advice.
Take care of your oral hygiene and treat cavities.
The product should not be reused because this can cause cross contamination.
Polymeric tracheal/bronchial stent, non-bioabsorbable, sterile
A sterile non-bioabsorbable tubular device intended to be implanted into the trachea and/or a bronchus/bronchiole to maintain luminal patency, typically used in cases of obstructions/stenoses, fistulae, tumours, scarring, surgical resection and anastomosis, or pulmonary transplantation. It is made entirely of a synthetic polymer(s) [e.g., silicone] and may have various designs (e.g., semi-soft continuous tube, covered or non-covered mesh structure, straight or branched configuration) intended to conform to the endotracheal/endobronchial surface. It may be expandable in situ (e.g., self-expands) and disposable devices intended to assist implantation may be included.
46977