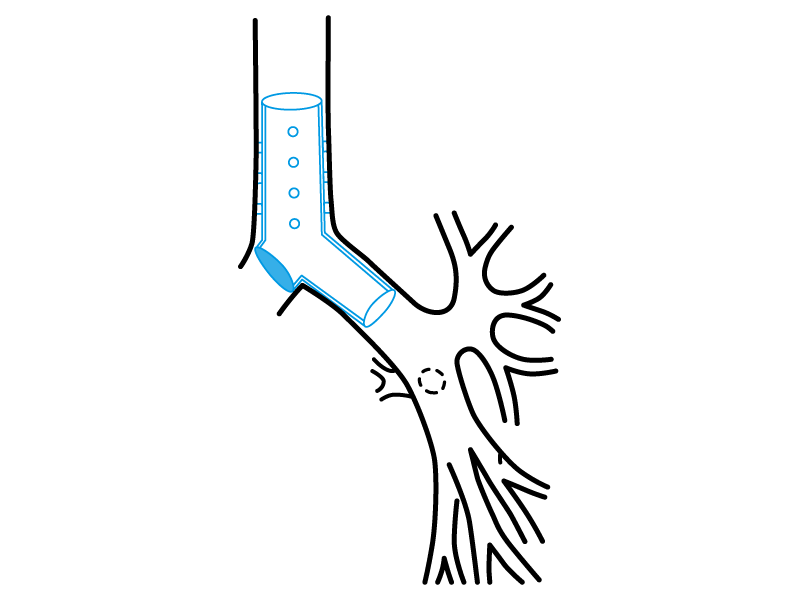
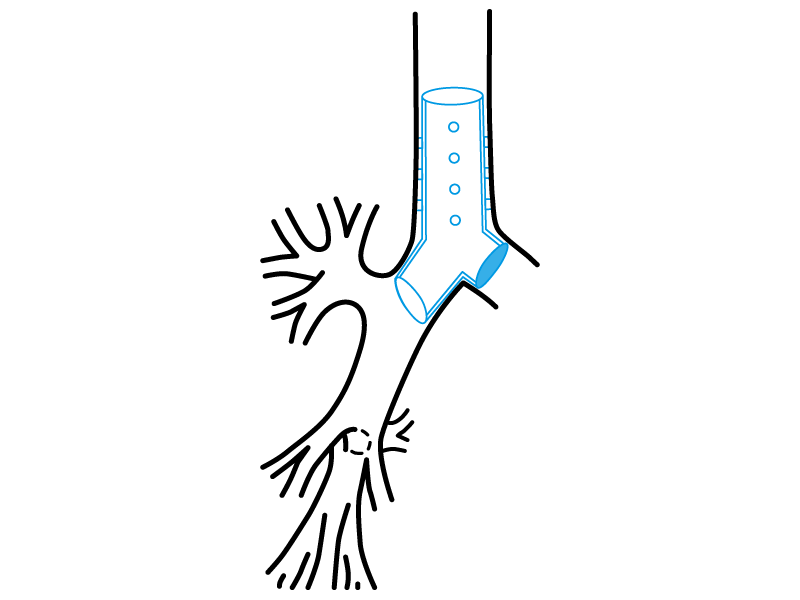
Stening® Occluded “Y” Stent
Code SYO

Description
It consists on a tracheo-carino-bronchial “Y” stent that has one of its bronchial branches completely occluded on its origin.
Thereby, the stent satisfies a special function, allowing the ventilation from the healthy lung on patients with a postsurgical bronchopleural fistula or from other aetiologies and that require mechanical respiratory assistance.
The occluded branch impedes the air flow loss through the vast communication with the pleural cavity.
Indications:
- A right or left bronchopleural fistula, from any aetiology, with or without the MRA (Mechanical Respiratory Assistance) need
- A bronchopleural fistula accompanied by empyema on patients with a tube drainage or buleau
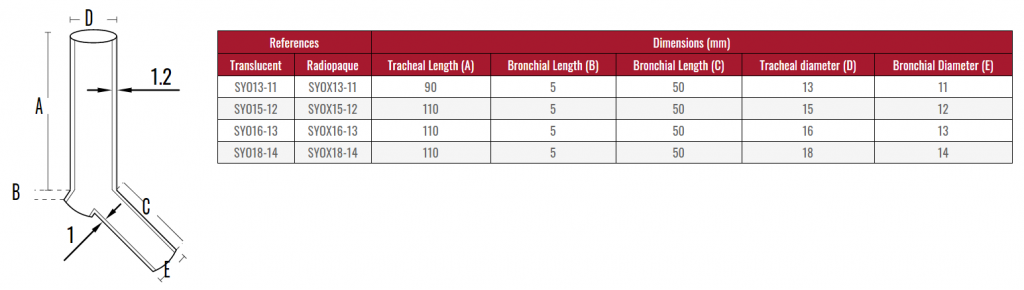
Due to the characteristics of the production process, the
measurements of the devices can vary by +/- 2%
- Medical grade silicone
- Bevelled edges to prevent granulomas
- Spur system to prevent migration
- Removable
- Surface of maximum softness to avoid adherence of secretions
- Transparent or Radiopaque
The manoeuvres needed for the implant of the standard Stening “Y” are described below, although it is not essential to use the special “Y” stent forceps, since this device has only one bronchial branch. The implant will be carried out then by introducing the stent inside a tracheoscope, to, afterwards, intubate the airway with the set. When loading the stent into the endoscope, the bronchial branch should be oriented in the direction that allows it to occupy in the airway the source bronchus or the affected stump. Once the patient is intubated, advance inside the trachea until you approach to the carina. The stent will then be pressed with a clamp, in order to force it to leave the tracheoscope and remain lodged in the trachea. The final position can be adjusted with the same forceps, so that the only bronchial branch of the stent is lodged within the chosen bronchus source.
Implant:
The process will take place under general anaesthesia. This kind of implants must be carried out by experienced staff. The stent can be fitted on a special forceps that is used for “Y” prostheses’ implant.
Lubricate the forceps’ margin with lidocaine gel. Introduce it inside de stent in order to make its valves penetrate into its bronchial branches (see the photo on previous page). Ventilate the patient with oxygen until you reach the higher saturation possible. After that we will proceed to the patient extubation by removing the tracheoscope from the airway. Immediately, and with the laryngoscope help, the forceps will be guided towards the trachea. When you close the forceps’ valves the bronchial branches from the stent will join, and, in this position, it will pass through the vocal chords towards the trachea. The manoeuvre will continue to displace the forceps-stent assembly inside the trachea until it approaches to the carina. When the edge of the forceps-stent assembly is close to the tracheal carina, the valves must open kindly so that they can notice the prosthesis arrival to the tracheal bifurcation.
The use of a special forceps for the introduction of the “Y” stent is not necessary, because this device has an only bronchial branch. The implant will be carried out by introducing the stent inside a tracheoscope to intubate the airway with it. At the moment of charging the endoscope, the bronchial branch must be oriented in a direction that permitted it occupy the source bronchus or affected stump.
Once the patient is intubated, advance through the trachea until you reach the carina. Then the stent will be pushed with a forceps to expel it from the tracheoscope and to stay housed in the trachea. Its final position can be adjusted with the same forceps, so that the only bronchial branch stay housed inside the chosen source bronchus.
Removal:
First we will proceed to the intubation with the tracheoscope.
The extraction is easier.
The stent must be taken by its proximal edge with a strong forceps to remove it kindly by pulling the forceps and removing the prosthesis at the same time you remove the tracheoscope.
This device application supposes a very critical condition of the patient; a respiratory assistance is required usually at the intensive care unit. Therefore, the care of the stent consists in frequent aspirations and a humidified airway, with the purpose of reducing the production and accumulation of secretions. It is a care usually provided in these hospitalization units.
The product should not be reused because this can cause cross contamination.
A sterile non-bioabsorbable tubular device intended to be implanted into the trachea and/or a bronchus/bronchiole to maintain luminal patency, typically used in cases of obstructions/stenoses, fistulae, tumours, scarring, surgical resection and anastomosis, or pulmonary transplantation. It is made entirely of a synthetic polymer(s) [e.g., silicone] and may have various designs (e.g., semi-soft continuous tube, covered or non-covered mesh structure, straight or branched configuration) intended to conform to the endotracheal/endobronchial surface. It may be expandable in situ (e.g., self-expands) and disposable devices intended to assist implantation may be included.
46977