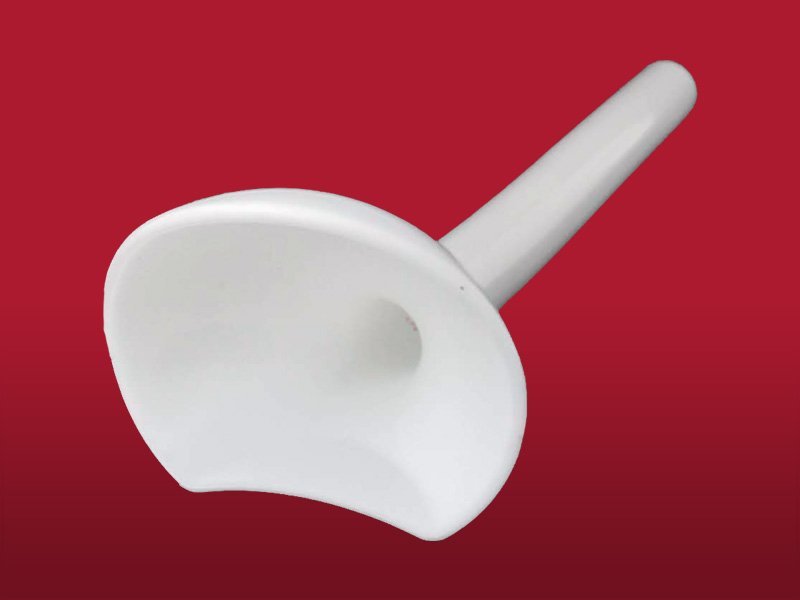
Pharyngeal Tube
Code TF12

Description:
It is made out of flexible silicone and is useful in a wide range of situations.
Its apical end is progressively enlarged, in this way the anterior edge maintains contact with the base of the tongue, while the posterior one rests on the pharyngeal wall. In that way, the saliva is stored in the oropharynx leading to oesophagus, preventing the maceration of the tissues and avoiding the aspiration of the saliva towards the airway.
A nasoenteral tube for feeding can be inserted through the Pharyngeal Tube.
Indications:
- Secondary fistulas to laryngectomy, radiotherapy, neoplastic conditions, caustic ingestion
- Orcutaneous or pharyngocutaneous, traumatic fistula
- Head and neck oncological surgery
- Esophageal stenosis
- Oesophagus carcinoma
Stening® provides detailed instructions for each device, including insertion and removal techniques, precautions and postoperative cares.
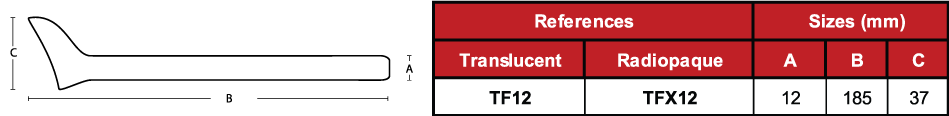
Due to the characteristics of the production process, the
measurements of the devices can vary by +/- 2%.
- Medical grade silicone
- Bevelled edges to prevent granulomas
- Removable
- Surface of maximum softness to avoid adherence of secretions
- Transparent or Radiopaque
The introduction of the pharyngeal tube should be done under general anaesthesia. It can be cut up to the length that is considered appropriate for the case. A laryngoscope will be used to have convenient access to the larynx and adequate vision. With the help of a hypopharyngoscope and a long forceps, take the tube by its distal end to lead it into the oesophagus, until the proximal cup is at the laryngeal level. Previous oesophageal dilatation may be necessary. After its introduction it can be fixed with a percutaneous point.
Perform a periodic check-up carried out by the physician.
The product should not be reused because this can cause cross contamination.
A sterile, non-bioabsorbable, expandable, tubular device intended to be implanted into the oesophagus to temporarily maintain luminal patency in strictures and achalasia, seal fistulas, and/or to treat other lesions (e.g., anastomotic leaks). It is a mesh structure, which may or may not be covered/lined, and is made entirely of a synthetic polymer(s) [e.g., silicone]. It is typically expanded in situ (e.g., self-expands) and disposable devices intended to assist implantation may be included.
43684