Stening® Solid Stent MS03
Code MS03
Description
Stening® Solid Stent made out of silicone and intended for bronchial occlusion in the treatment of different bronchopleural pulmonary affections such as the bronchopleural fistula and persistent air loss in pneumothorax cases that cannot be treated with conventional surgery.
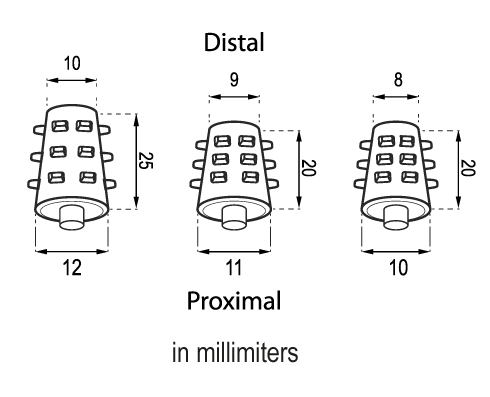
The Solid Stent is presented in three different sizes: MS810, MS911 and MS1012.
It is radiopaque (white or pale yellow).
Stening® provides detailed instructions for use with each device, including insertion and removal techniques, precautions, and postoperative care.
- Bronchopleural fistula.
- Suture failure in a stump after lobar pulmonary resection.
- Occluded bronchial treatment.
- Hemoptysis.
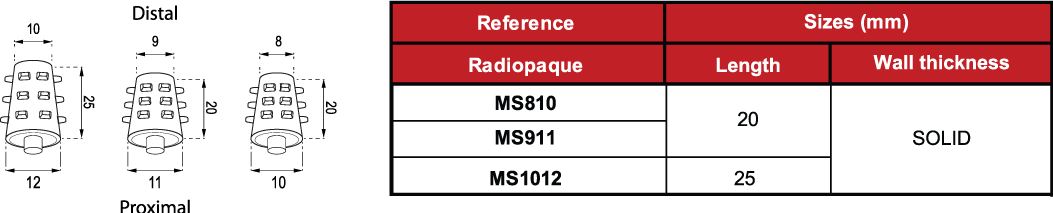
The measures of the devices are subject to a variation of +/- 2%.
- Medical grade silicone
- Bevelled edges to prevent granulomas
- Spur system to prevent migration
- Removable
- Surface of maximum softness to avoid adherence of secretions
- Maintain the moisture of secretions, whenever they appear, by taking nebulisations frequently with a warm isotonic saline solution.
- Perform a periodic check-up following your doctor’s advice.
Warning: This type of device may suffer an unexpected displacement. This will depend on the failed choice of stent caliber in relation to the bronchial or fistulous orifice where it has been placed, as well as the unpredictable individual reaction of the tissue that acts as support in the implant area. A device that has migrated may be removed by coughing or accidentally lodged in another bronchus in the same or adjacent lung, resulting in unwanted bronchial occlusion that may lead to severe ventilatory insufficiency and subsequent death.
A displacement of the occlusion device towards the pleural cavity in cases of post-pneumonectomy fistula is possible, and also its spontaneous elimination through an existing pleurocutaneous window.
An anomalous position may be suspected by the appearance of a sustained cough. The devices are radiopaque and their position can be identified on chest radiography. A displaced device must be removed by the interventional physician.
The product should not be reused because this can cause cross contamination.